Early Cancer Detection
Role
Principal UX Designer
ctDNA Product Page
Mutation Profiler Product Page
The Cancer Problem
Currently the only way to know that a tumor exists (or an excised tumor is coming back) is to visually confirm a tumor is a large enough mass to be seen by the human eye. This is problematic as there is not any early warnings available, when you can do something about cancer.
Solution
Next-generation sequencing to detect circulating tumor DNA (ctDNA) via a small blood sample (aka “liquid biopsy”) is a minimally invasive method for tumor detection, genotyping (find genetic mutations), early detection, and monitoring therapeutic response.
Software Problem (What I am actively working on)
The assay kit is just a small piece of this ctDNA detection puzzle. Processing the data that comes out of the lab work and modeling what the lab users did in the assay (entering pertinent data), sequencing the output of the assay, then analyzing that sequencing data into actionable, real results is complicated.
Solution
The aim of the software that is packaged with the AVENIO ctDNA assay is to ease just these issues and run from the lab to the end report without issue. The software is designed to tie into the assay steps so you can enter the data and get results with ease of a modern interface.
My role in the AVENIO oncology software product
As the lead designer on the AVENIO Oncology Analysis Software project, it was my job to envision what the product will look like, and understand how the users would use it effectively. Then convey and create a shared understanding of the software to the product manager, stakeholders, verification and validation, and engineers. I accomplished this by:
1. Initial UX Research - Establishing the user's mental model of how software processing Oncology samples should flow (via interviews, shadowing, user testing, and other UX research methods).
2. Detailed UX Research - Understand how users currently understand and interpret results by going into their labs and learning their existing systems and lab structure.
3. Prototyping - Building iterative prototypes of all aspects of the system, then testing and learning from each interaction.
4. UX process and communication - After the research and prototypes have been solidified, I then broke down the product into sections and translated design into what I call "Design Documents" to inform the engineers exactly what to build down to the pixel level. This process has taken a few years of vetting to perfect, but it has led to a very harmonious sprints with very little thrashing during the sprint itself for efficiency.
In the end, I am responsible for how our users interact with the product. From entering the correct information, to get back reliable and easily understood results. The post-launch is always an opportunity to learn and iterate further, but customers are always delighted to use the product.
Overview of what the ctDNA product is (some context)
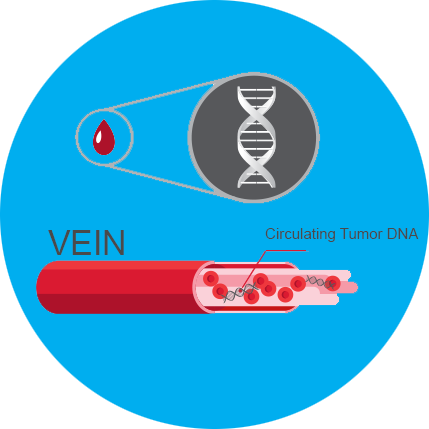
What is ctDNA?
Circulating tumor DNA (ctDNA) is tumor-derived fragmented DNA in the bloodstream that is not associated with cells. When you isolate the tumor ctDNA (knowing what kind of cancer you are looking for) it can be used as a “liquid biopsy” in the form of blood draws - and may be taken at various time points to monitor tumor progression throughout the treatment regimen. As this technology grows it can be used as a cancer diagnosis or early screening. I am very excited about future use of this early screening method.
TL;DR: Lots of things shed DNA in your blood, including tumors if you have one. You can isolate that DNA and amplifiy it
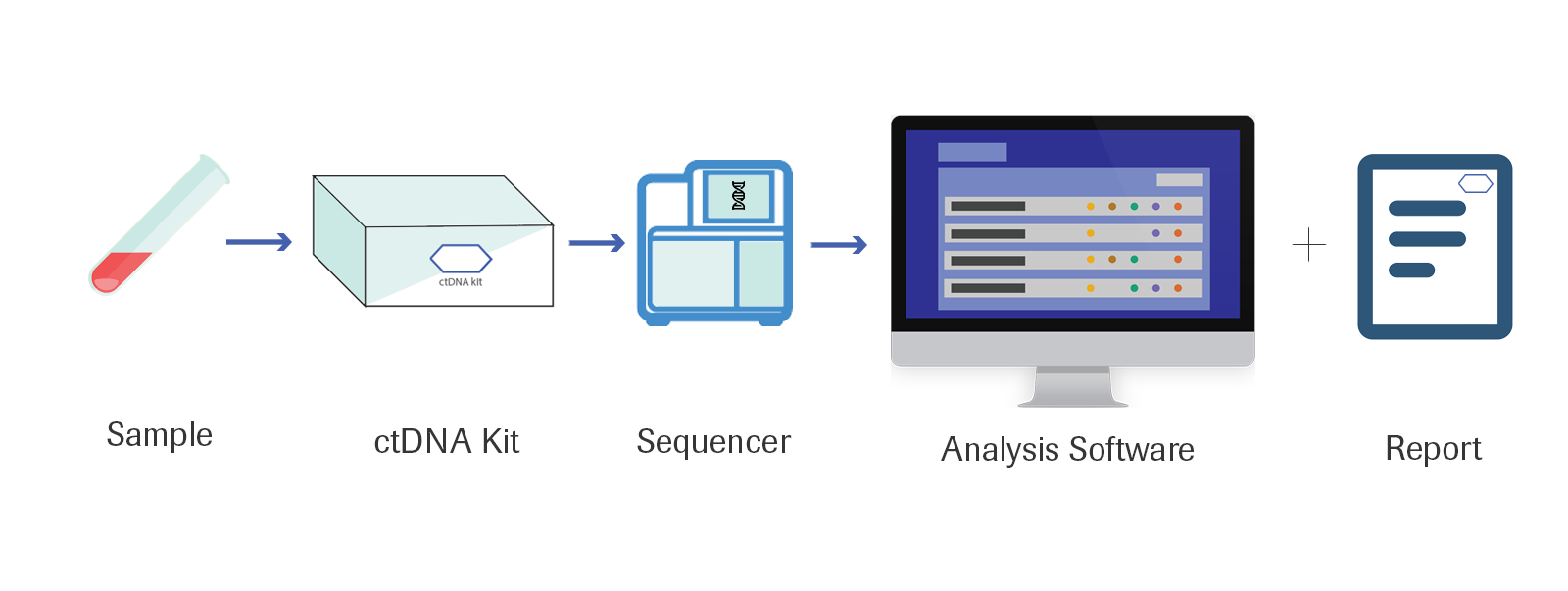
The overall workflow of ctDNA
1. Input 4ml of plasma from a subject's blood.
2. Run the ctDNA assay kit on the sample to isolate the ctDNA.
3. Sequence the output from the kit.
4. Enter the information into the software and import the sequencing file and the AVENIO software calls all variant information for your subject and outputs information in an easy to use UI, which you can also download a correlating PDF report.